Faster results for in vitro corneal permeability studies
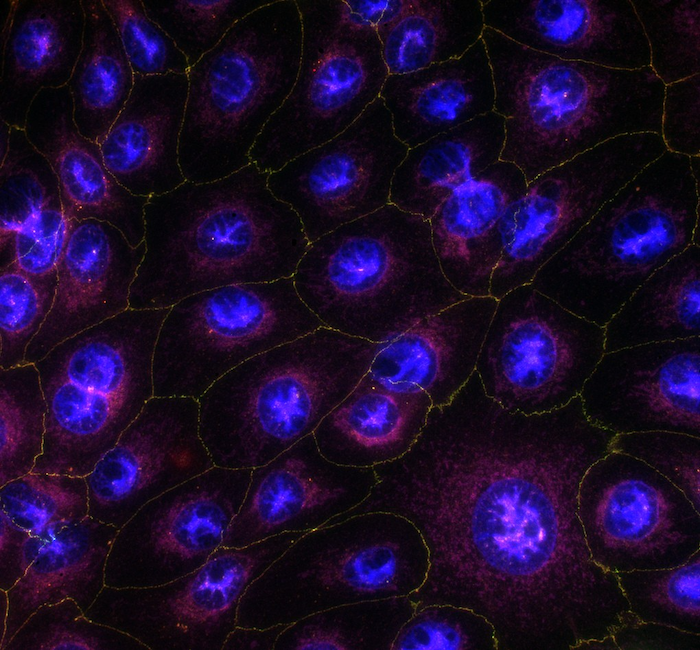

Corneal epithelial cells form the outermost layer of the cornea, the transparent part of the ocular surface that protects the delicate structures within the eye. The cornea functions as a window, regulating the entry of light, while its multilayered structure also serves as an effective barrier that limits the penetration of ophthalmic drugs. In the early stages of ocular drug development, it is therefore crucial to determine whether a novel compound administered topically as eye drops can penetrate the cornea, reach the target ocular tissues, and achieve its intended therapeutic effect.
Corneal permeability testing can be conducted in vitro using human-derived corneal epithelial cells cultured under laboratory conditions. In the past, such studies were commonly performed on excised rabbit corneas. Since then, various 2D cell-based models utilizing epithelial cells from different species have been developed. Currently, significant scientific efforts are focused on advancing 3D organotypic and organ-on-a-chip models that better mimic the structural and functional characteristics of the human cornea.
At Experimentica, we have advanced in vitro corneal permeability testing to a new level. Our human cornea organ-on-a-chip model, developed on the AKITA® platform, demonstrates permeability profiles for passively-diffusing compounds comparable to those observed in previously established models (ARVO 2025 poster1). Notably, the accelerated maturation of the epithelial cells significantly reduces the overall time required to obtain final results.
This innovation offers tangible benefits for our clients, including expedited time to study start (reduced timelines from 6 weeks to 3 weeks), thereby facilitating more efficient project progression. Additionally, using this technology requires reduced volume of test article solutions, which is a crucial factor in the early phases of drug development. By leveraging this new technology, our clients can more easily and cost effectively evaluate compound permeability early on, helping them make evidence-based decisions about which compounds to advance.
Director, In Vitro Pharmacology
There is a critical need for the continued development and rigorous validation of human-derived in vitro models capable of delivering robust and predictive data on human-specific responses. In alignment with the 3R principles (Replacement, Reduction, and Refinement) we have transitioned our permeability testing from traditional excised rabbit cornea experiments (ex vivo) to more ethical and innovative in vitro models. Initially adopting human corneal epithelial cells (HCE-T) cultured in inserts and combined with collagen hydrogel2 we have now advanced to a sophisticated cornea-on-a-chip platform1, developed through our own scientific expertise. This evolution not only enhances the relevance of our studies but also significantly reduces animal use, underscoring our commitment to scientific excellence and animal welfare.
1 Litvinavičiūtė D, Vergun O, Nguyen TH, Mosser S, Hakkarainen JJ. Human cornea-on-a-chip – opportunities and challenges. Poster presentation ARVO 2025 Annual meeting, May 5, 2025, Salt Lake City, Utah. https://iovs.arvojournals.org/article.aspx?articleid=2806432
2 Žiniauskaitė A, Cėpla V, Jelinskas T, Eimont R, Ulčinas A, Aldonytė R, Valiokas R, Kalesnykas G, Hakkarainen JJ. Introducing an Efficient In Vitro Cornea Mimetic Model for Testing Drug Permeability. Sci. 2021; 3(3):30. https://doi.org/10.3390/sci3030030
Please contact our Chief Business Officer!
Check out our latest news and activities
All News
Copyright: Experimentica Ltd. 2026