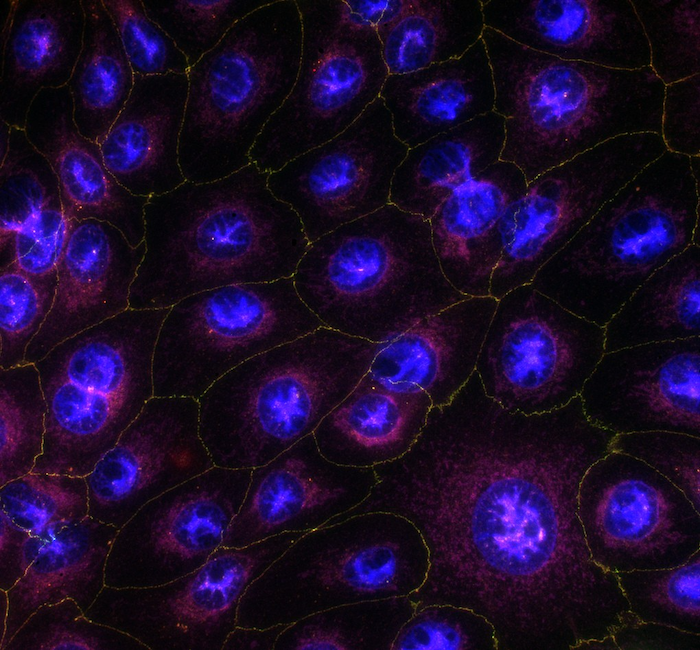
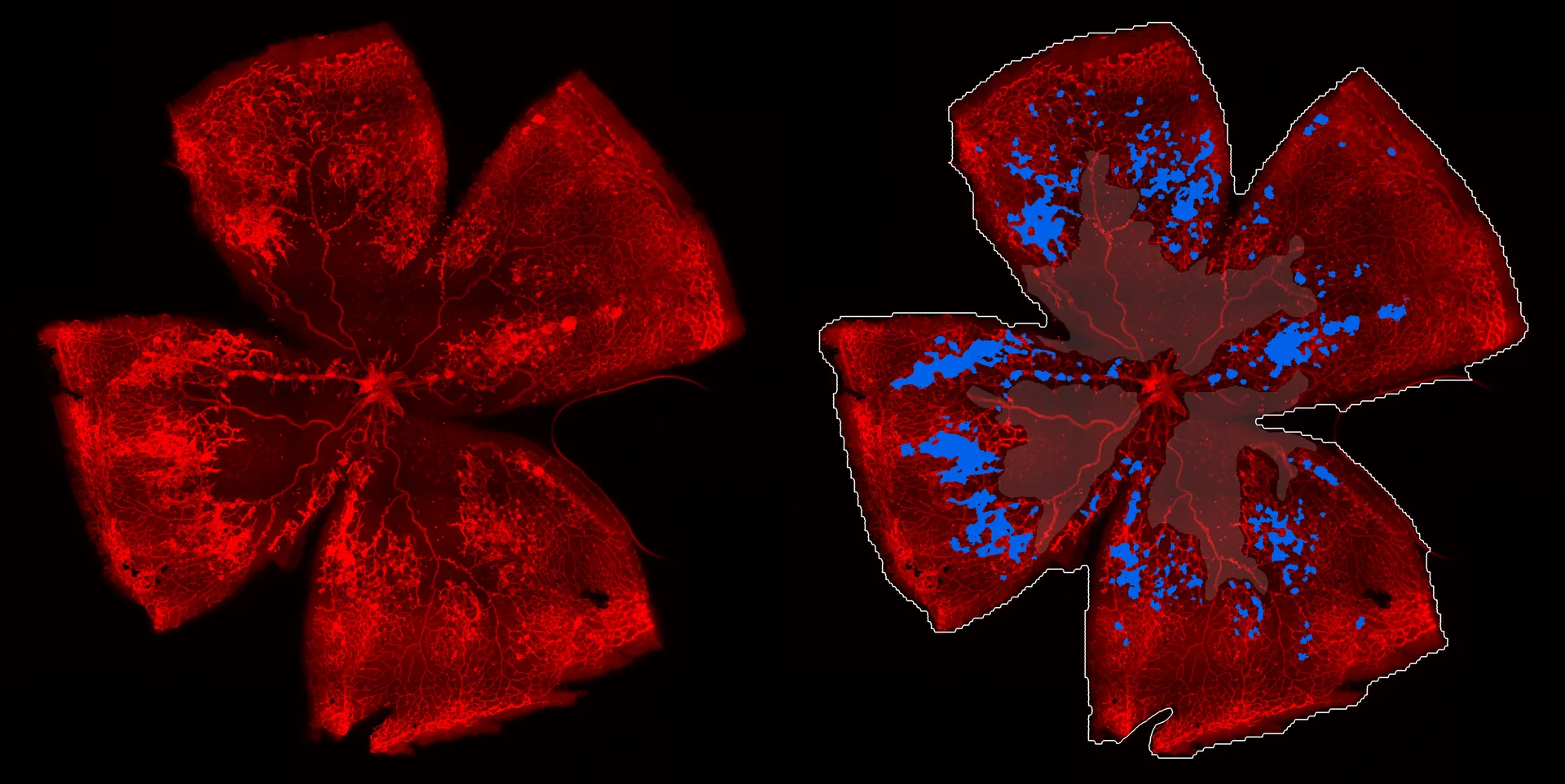
Glaucoma, a leading cause of blindness and visual impairment worldwide, is characterized by progressive loss of retinal ganglion cells (RGCs) and damage to the optic nerve. Neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF), have been extensively studied for their potential to promote RGC survival and axonal regeneration. However, BDNF's efficacy is restricted by receptor desensitization, while CNTF alone often induces inflammation and gliosis, limiting its therapeutic potential. This study was aimed at evaluating if a combination of BDNF and CNTF offers synergistic effects, providing superior neuroprotection as compared to either factor alone.
Learn more